TRAINING PROGRAM ON PATIENT SAFETY RESEARCH
In order to improve and strengthen research excellence of the ICM-UT in the field of patient safety research among the early stage researchers (ESRs) and staff the PATSAFE partners have developed a unique training curriculum.
This curriculum consists of 13 courses divided between three modules. The training started in March 2020 and will end by December 2021. Since March 2020, the training activities were significantly affected by the corona pandemic. Therefore, all contact learning was cancelled and the training program was redesigned for e-learning. The e-learning is carried out in the Moodle e-learning environment of the University of Tartu.
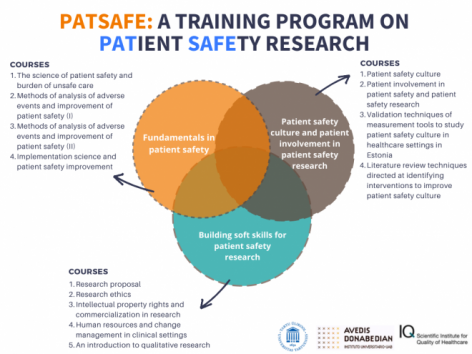
Module 1. Fundamentals in patient safety
Coordinator: Carola Orrego, Avedis Donabedian Foundation, Spain
March 4th-5th, 2020 (workshop in Tartu). September 21st, 2020 – January 31st, 2021 (e-learning).
Objectives:
- To describe and raise awareness of why patient safety has emerged as an important issue in healthcare
- To identify the impact of adverse events at different levels of care (including the extent of specific adverse events such as nosocomial infection, events related to medication and others)
- To describe the methods used for epidemiological studies of adverse events
- To identify how to define the severity and preventability of adverse events
- To describe existing taxonomies on patient safety and their potential applications in research
- To describe basic definitions and foundational concepts, including human factors and organizational theory
- To list and discuss major types of errors and related latent conditions
- To explain how system design and the influence of human factors affect healthcare outcomes
- To identify research gaps in the area of the burden of unsafe care
Content:
- Patient safety as a dimension of quality of care
- Epidemiology and nature of adverse events
- Methods for studying the impact of adverse events
- Identification of specific adverse events (e.g., surgical adverse events, nosocomial infections, medication, falls)
- Basic concepts of patient safety
- Errors and the human factors in the patient safety science
- Taxonomy of patient safety
Learning outcomes
The students who have completed the course will be able to:
- Prioritize patient safety issues, focusing on the main adverse events in their work field or clinical setting,
- Use a standardized vocabulary and concepts as an essential requirement for effective patient safety research and improvement (including the need for distilling complex interventions into their essential components, facilitating comparisons and promoting clear communications with different stakeholders),
- Introduce a non-punitive approach to research activities and improvement efforts related to patient safety,
- Recognize research areas related to the burden of unsafe care in their local area or clinical setting.
January 11th to March 31st, 2021.
Objectives:
- To describe "reactive" methods for analyzing adverse events in clinical settings,
- To describe the concept of sentinel adverse events,
- To describe the methodology of root cause analyses identifying latent conditions and root causes,
- To describe the state of the art and research opportunities for reporting systems for adverse events,
- To describe the use of trigger tools in clinical settings,
- To describe the main patient safety indicators and their application,
- To identify the main research gaps in the area of adverse events and root cause analysis,
- To perform techniques of full disclosure to patients once an error has occurred.
Content:
- Root cause analysis,
- Sentinel adverse events,
- Reporting systems,
- Trigger tools (including global, surgery, ICU, medication),
- Patient safety indicators,
- Disclosure of adverse events to patients.
Learning outcomes:
The students who have completed the course will be able to:
- Investigate root causes and suggest potential solutions to address problems related to adverse events,
- Recognize research areas related to reactive methods for analysing and patient safety improvement in their own clinical area.
This course will be launched in the first half of 2021.
Objectives:
- To describe “proactive” methods of risk and hazard analysis,
- To identify high-risk processes and describe the concept of process reliability,
- To describe the methodology of Healthcare Failure Mode and Effects Analysis (HFMEA) and conduct analyses of items with the greatest severity,
- To describe alternative/complementary methodologies emerging from the field of safety-critical engineering,
- To understand how to address processes to prevent or mitigate risks and/or protect patients from the effects of the failure,
- To understand the importance of creating environments for safe care,
- To identify the main research gaps in the area of risk assessment and process reliability.
Content
- Prospective hazard analysis techniques,
- HFMEA,
- Strengths and weaknesses of HFMEA,
- Systematic Human Error Reduction and Prediction Approach (SHERPA),
- System Theoretic Process Analysis (STPA) / System,
- Theoretic Accident Model and Processes (STAMP),
- Process reliability,
- Tools and methods for patient safety improvement.
Learning outcomes
The students who have completed the course will be able to:
- Implement proactive methods to identify and address the main risks and hazards in their own clinical practice
- Propose research areas related to proactive methods to identify adverse events and improve patient safety.
This course will be launched in the second half of 2021.
Objectives
- To describe the top evidence-based patient safety practices,
- To identify core concepts and challenges of implementation research in clinical settings,
- To describe differences between quality improvement and implementation research,
- To assess the appropriateness and effectiveness of existing implementation strategies to implement evidence-based safety practices,
- To design research projects on implementation, including how to define outcomes, evaluate effectiveness and prepare scaling-up processes,
- To describe how to use mixed methods in implementation research considering the impact of the healthcare context,
- To describe the concept of healthcare microsystems; and how healthcare professionals can be effective as part of a larger whole.
Content
- What is implementation research,
- Implementation research vs quality improvement,
- Designs used to evaluate implementation in real practice,
- Top evidence-based patient safety practices,
- Context analysis,
- Implementation strategies,
- Tailored and adapted strategies to implement evidence-based practices in real world settings.
Learning outcomes
The students who have completed the course will be able to:
- Translate research evidence to improve the safety of patient care
- Select implementation strategies tailored to the local context
- Propose research areas related to implementation research of evidence-based safety practices.
Module 2. Patient safety culture and patient involvement in patient safety research
Coordinator: Hilly Calsbeek, IQ-HC, The Netherlands
October 5th, 2020 to March 31st, 2021
Objective: to develop understanding about the importance of safety culture for patient safety and methods to measure and improve safety culture.
Content
What is safety culture in healthcare?
- Organizational culture in healthcare (conceptualization of culture).
- Culture or climate?
- Safety culture in healthcare.
How does safety culture influence patient safety?
- How different elements of safety culture are related to patient safety.
- Safety culture and patient safety outcomes.
- Safety culture as a barrier or facilitator for improvement in patient safety.
Assessing patient safety culture
- Using qualitative or quantitative methods for assessing patient safety culture?
- Quantitative instruments to measure safety culture
- Qualitative methods to explore safety culture
Improving patient safety culture
- Can culture be changed?
- Development phases to a safety culture.
- Examples of methods to improve patient safety culture.
Learning outcomes
The students who have completed the course:
- Can explain why safety culture is important for patient safety and how culture can affect patient safety.
- Can describe examples of methods and instruments of measuring safety culture.
- Can describe examples of interventions to manage and improve safety culture.
The course started at November 2nd, 2020 and will end at March 31st, 2021
Objective: to develop understanding about the importance of patient involvement in patient safety and research, and methods to measure and improve patient involvement.
Content
Patient involvement in patient safety
- The concept of patient involvement.
- Levels of patient involvement.
- Patient involvement in patient safety.
Assessing patient involvement in patient safety
- Qualitative methods to explore patient involvement in patient safety.
- Quantitative methods to measure patient involvement in patient safety.
Improving patient involvement in patient safety
- Factors that influence patient involvement in patient safety.
- Methods to improve patient involvement in patient safety.
Patient involvement in research
- Why involving patients in research?
- Ways to involve patients in research (patient related outcomes measures, participatory research designs, quality improvement initiatives).
- Evaluating patient involvement in research.
Learning outcomes
The students who have completed the course:
- Can explain why patient involvement in patient safety is important.
- Can describe examples of strategies for measuring patient involvement in patient safety.
- Can describe examples of strategies for improving patient involvement in patient safety and factors that hinder or facilitate patient involvement in patient safety.
- Can explain the importance of involving patients in (patient safety) research.
March 1st to December 31st, 2021
Objectives
- To develop the skills to validate measurement tools to study patient safety culture in healthcare settings in Estonia,
- To use validated measurement tools on patient safety culture in healthcare settings in Estonia.
Content
- The importance of validating instruments
- Different validation methods
- The process of validating instruments
- Performing and distributing a validated tool on patient safety.
Learning outcomes
The students who have completed the course:
- Understands why validation is important for using measurement tools
- Knows the different types of validation methods
- Is able to validate a measurement tool on patient safety culture for a healthcare setting in Estonia
This course will be launched in the second half of 2021.
Objectives:
- To describe the different kinds of literature review methods and understand the different steps in performing a systematic review,
- To develop the skills to perform a review on safety culture interventions in relation to patient safety.
Content
- The importance of literature reviews;
- Different kinds of review methods;
- Steps in performing a systematic review.
Learning outcomes
The participant who has completed the course:
- Understands why literature reviews are important;
- Understands the different literature review methods;
- Can describe the steps in performing a systematic review;
- Is able to perform a systematic review on safety culture interventions in relation to patient safety (Only applies to participants who choose to perform and submit a literature review).
Module 3. Building soft skills for patient safety research
Coordinator: Mari Kangasniemi, ICM-UT, Estonia
March 4th-5th, 2020 (workshop in Tartu), e-learning between May-August, 2020.
Objective: To deepen knowledge about the research proposal (e.g. aims, structure and funding opportunities)
Content
- The structure of research proposal.
- Formulation of problem identification and formulation of research questions on patient safety
- Principles to formulate a research proposal for different purposes, including methodological attachments.
- Timetable and budgeting of research projects.
- National and international research funding sources.
Learning outcomes
The students who have completed the course:
- is able to formulate and develop research questions on the area of patient safety;
- is familiar with the structure of a research proposal in general;
- is able to write a research proposal in the field of patient safety according to its targets (e.g. grant application, for ethical committee);
- is aware of the principles of time allocation and budgeting of a research project;
- is familiar with channels in the field of patient safety or quality of care to search for national and European funding sources for a study on patient safety;
- is familiar with the structure, sections and critical success factors of the EU funded research proposals.
March 1stto April 30th, 2021.
Objective: To deepen knowledge of research ethics, to be aware and recognize them in health research, improve abilities for applying research ethics permission.
Content
- Ethical principles of health research.
- Informed consent and research documents.
- Researcher’s responsibilities in research project.
- The process of ethics review by ethics committee.
Learning outcomes
The student, who has completed the course:
- is aware of the principles and the main literature and documents of research ethics in health research
- acknowledges the research ethical questions in the individual research proposal
- is familiar the informed consent process with documents and researchers responsibilities
- is able to prepare application for research ethics committee
This course will be launched in the second half of 2021.
Objective: To get understanding of intellectual property rights as a part of health research and improve abilities to recognize commercial opportunities of health results findings.
Content
- Understanding of intellectual property rights as a part of health research.
- Recognition of commercial opportunities of health results findings.
Learning outcomes
The students who have completed the course:
- is aware of intellectual property rights and the main regulation for it
- is able to recognize the commercial opportunities of the individual study.
This course will be launched in the second half of 2021.
Objective: To deepen knowledge of the principles of the change management in clinical setting and abilities to identify challenges and opportunities when adopting new patient safety practices.
Content
- Implementation theories of novel practices.
- Patient safety sensitive challenging in the change management in clinical setting.
Learning outcomes
The students who have completed the course:
- is aware of the principles of the change management in clinical setting
- is able to identify the subject specific challenges when adopting change management in patient safety practices.
October 5th, 2020 to March 31st, 2021
Objective: to develop understanding and skills about performing qualitative research.
Content
The following topics will be addressed:
- What is qualitative research?
- Formulating a qualitative research questions.
- Collecting qualitative research data
o Sampling
o Methods of data collection
- Analysing qualitative research data
o Different approaches to analyse qualitative research data
o Practical steps in qualitative analysis
- Quality assurance in qualitative research
- Reporting qualitative research findings
Learning outcomes
The participant who has completed the course knows:
- the principles of qualitative research (and how it differs from quantitative research),
- different methods to collect qualitative research data,
- how to formulate a qualitative research question,
- how to systematically analyse qualitative data,
- how to assess the methodological quality your qualitative research
- how to report findings into a scientific journal.


